Fractional Exhaled Nitric Oxide
FeNO provides an excellent non-invasive, reproducible and reliable way to test for airway inflammation and demonstrate therapeutic responsiveness.
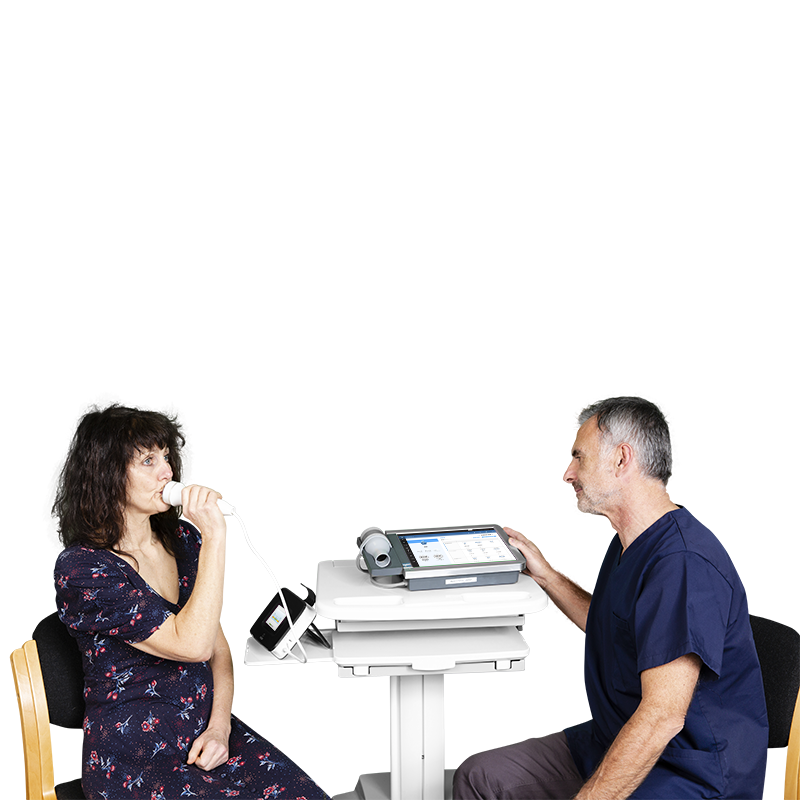
Fractional exhaled nitric oxide (FeNO) is an objective biomarker of airway inflammation1,2 providing a quantitative, non-invasive, simple and safe method of measuring airway inflammation. It is used for monitoring and assessing allergic asthma and other conditions with underlying inflammation such as chronic cough. In combination with other lung assessments such as spirometry it can be a valuable endpoint.
FeNO testing is offered as a module in Spirotrac® software on the COMPACT Medical Workstation. Vitalograph FeNO solutions utilise the NIOX VERO®, which provides a highly accurate measure of airway inflammation, with results automatically transmitted from the NIOX VERO to the COMPACT. Visit workflows may be customized in Spirotrac to align with your Clinical Trial protocol and meet study endpoints.
Our team of Respiratory Physiologist SMEs are on hand to advise and support with study design that includes FeNO assessment.
- Alving K, Malinovschi A. Basic aspects of exhaled nitric oxide. Eur Respir Mon. 2010;49:1-31.
- Dweik RA, Boggs PB, Erzurum SC, et al; on behalf of the American Thoracic Society Committee on Interpretation of Exhaled Nitric Oxide Levels (FENO) for Clinical Applications. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011;184(5):602-615.
Vitalograph’s FeNO assessment solution complies with the current ATS/ERS guidelines and includes:
- Integrated with COMPACT Medical Workstation.
- NIOX VERO + test kits and consumables.
- Study software customized to protocol requirements.
- Site training.
- Advice and support for study design including FeNO.