Spirotrac Software
Spirotrac® software is tailored for each study, centralizing endpoints collected from Vitalograph and third party devices on a single, user-friendly platform.
Spirotrac software offers sites a purpose built platform for subject and endpoint management. It is highly flexible and specifically tailored for each study protocol so that it includes only the necessary modules for the endpoints being collected for that study.
Configurable modules include Spirometry, ECG, DLCO, lung volumes, 6MWT, ePRO, eCOA, Blood Pressure, PCF, IFR, SNIP, MIP/MEP and FeNO.
Spirotrac is compliant with ATS/ERS guidelines. The software runs on the COMPACTTM Medical Workstation and is also used to manage Vitalograph and third-party devices required for the study.
Spirotrac® combines all the Vitalograph-collected protocol endpoints into one centralized software platform that is stored locally on the COMPACTTM workstation and transmitted to a single database. This makes it easy for site staff to navigate between assessments and manage subject data, and also for datasets to combine all assessments.
Because Spirotrac is tailored to each study it is possible to guide sites as much or as little as sponsors wish. Reporting is also fully flexible to the needs of sites and sponsors. In addition, Spirotrac reflects the visit schedule of the protocol and the specific alerts required for each trial. The software is designed to gather data not only from our full range of Vitalograph devices but also through third party devices which may be added as required for specific study needs.
Test Types Supported
- Spirometry: Single breath testing, multi-breath testing, tidal breathing. SVC, VC, FVC, MVV, IFR, PCF, Challenge Testing, Serial Spirometry, MIP/MEP
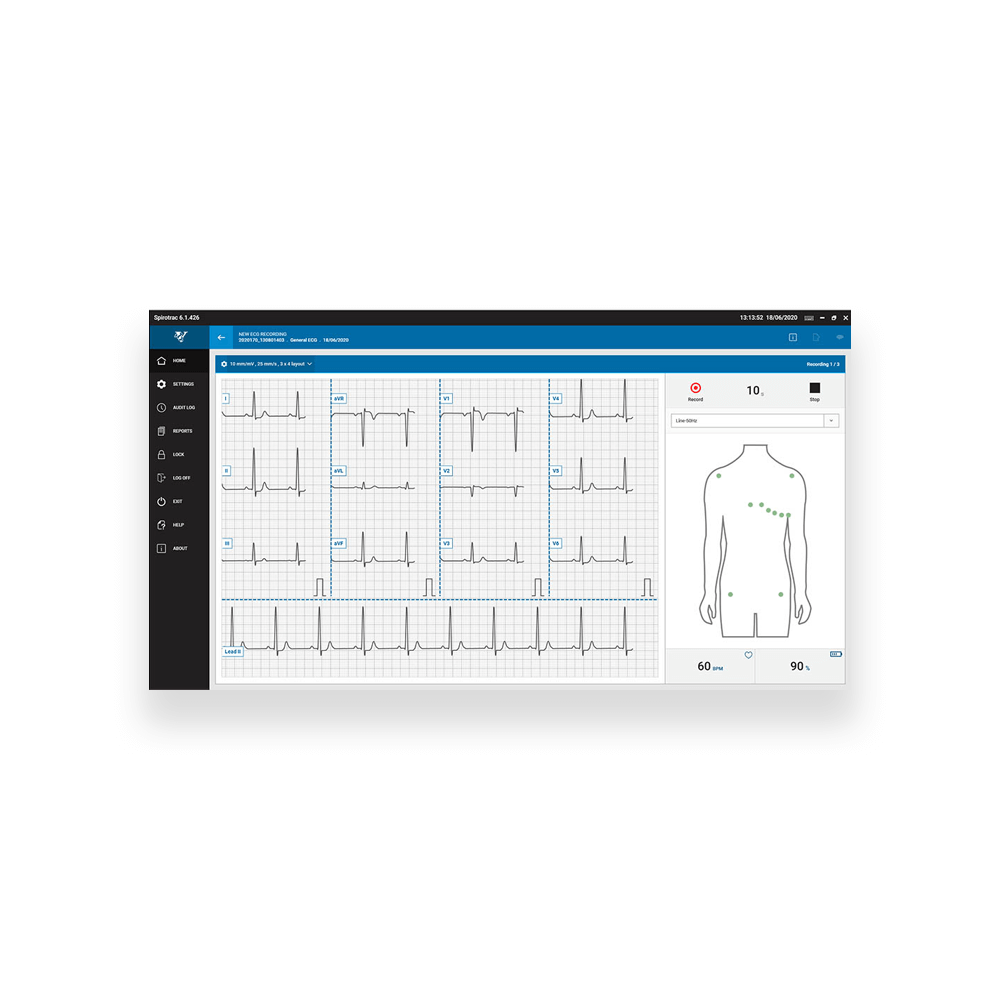
- ECG: 12 lead 10 second trace, H12+ Holter ECG
- SpO2 pulse oximetry including six minute walk test (6MWT) COPD assessment
- Blood pressure measurement
- Fractional exhaled nitric oxide (FeNO)
- DLco and Lung Volume capture
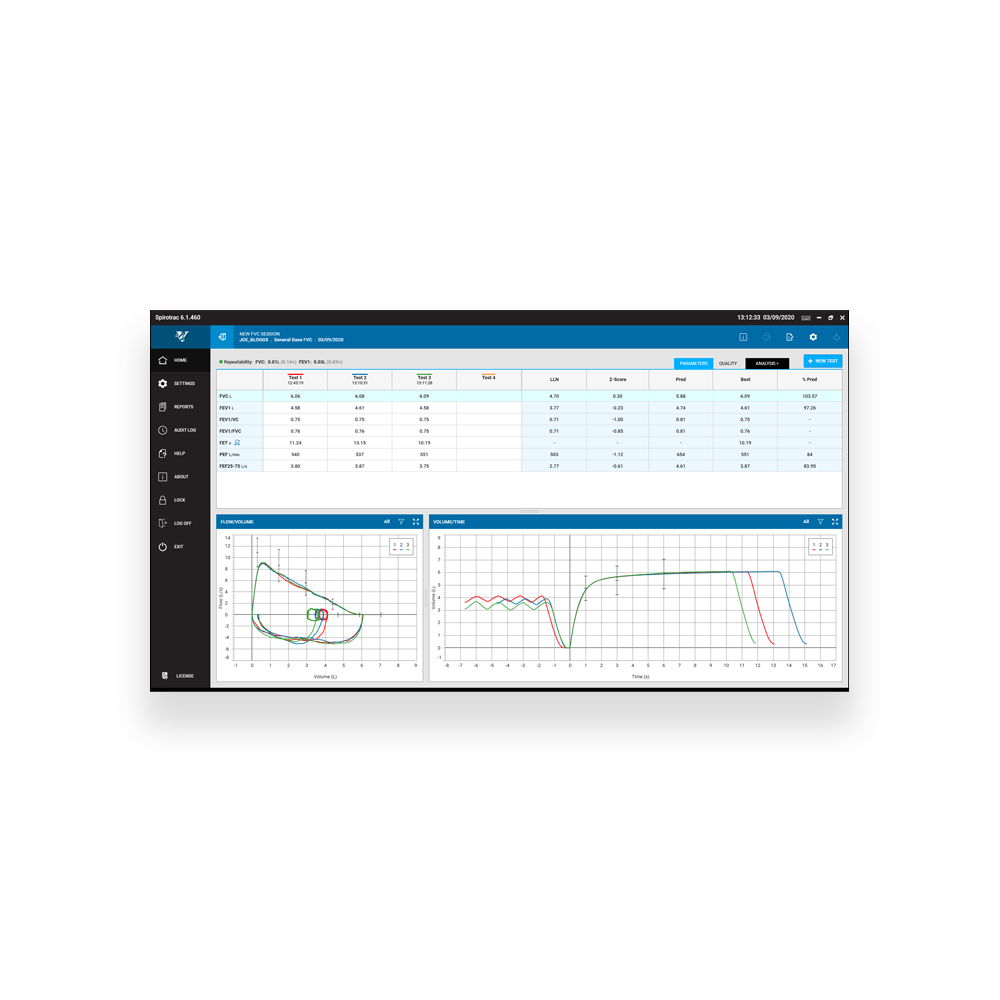
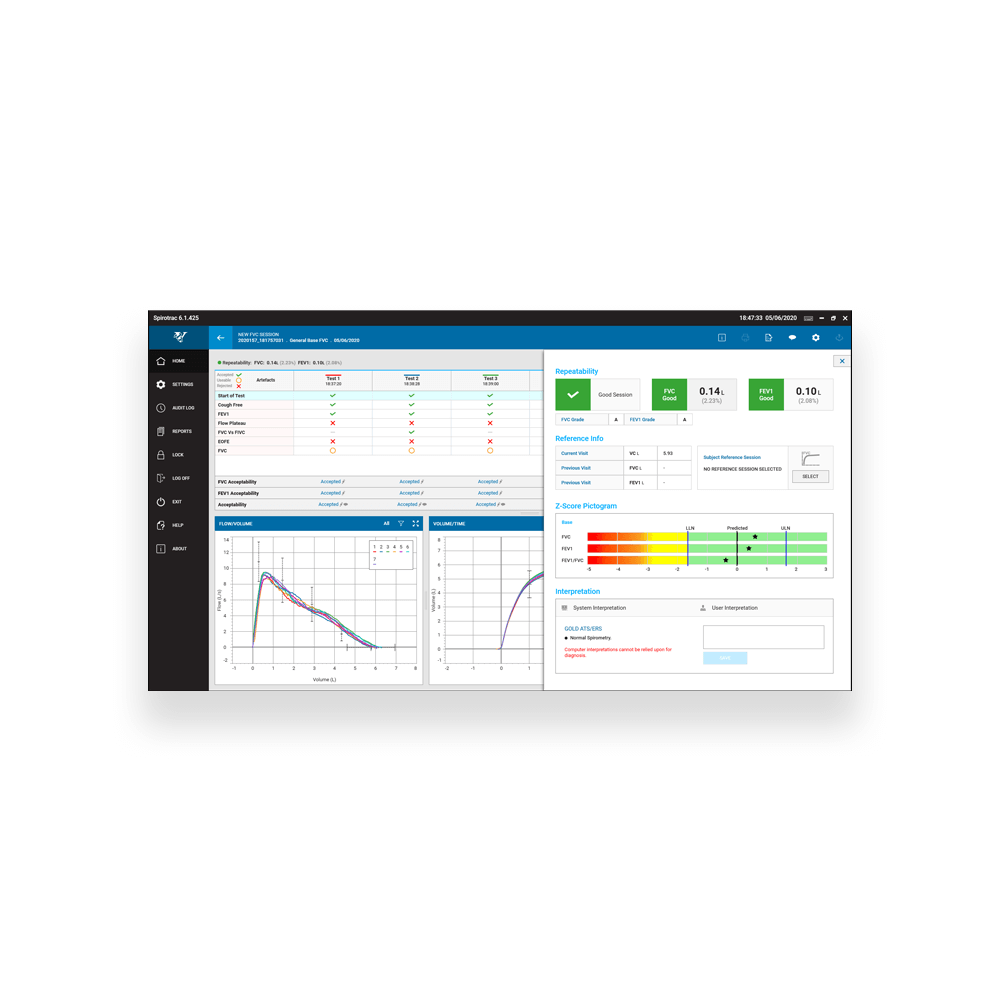
FVC Parameters
FVC, FEV1, FEV6, FEV1/FVC, FEV1/FEV6, FEV1 Ratio, PEF (L/s), PEF(L/min), FIVC, PIF (L/s) , PIF (L/min), EF25-75, TV, IRV, ERV, IC, EV, TExt, TPef, FET, FEV.5, FEV.5/FVC, FEV.75, FEV.75/FVC, FEV3, FEV3/FVC, FEV6/FVC, FEF0.2-1.2, FEF75- 85, FEF2575/FVC, FEF25, FEF50, FEF75, FEV1/PEF, FIF25, FIF50, FIF75, FIF50/FEF50, FIVC/FVC, FIV1, FEV1/FIVC, FIV1/FIVC, FIV1/FVC, FEF50/FIF50, MVVind, EV/FVC, FMFT, IVC, FEV1/IVC, FEFmax, EOTV , FEV1/HT2, FEV1/VC, FEV3/VC, RAWind, RV, FRC, TLC, Lung Age
VC Parameters
VC,SVC, IC, ERV, FRC, RV, TLC
MVV Parameters
MVV, VT, MVV Time, MVV Rate
ECG Parameters
HR[/min] Heart Rate
RR[ms] R-R interval
P[ms] Duration of the P wave
PQ[ms] Duration of the PQ interval
ST[ms] Duration of the ST segment
PR[ms] Duration of the PR interval
QRS[ms] Duration of the QRS complex
QT[ms] Q-T interval
QTc[ms] Q-T interval, corrected by the heart rate with options Fridericia, Framingham, Bazett and Hodges
Predicted Values Selectable
GLI, ERS/ECCS; NHANES, Polgar, Pereira, Berglund, Forsche, Gutierrez, Hedenström, Knudson, Crapo; Hsu; KNLW; Viljanen; SEPAR; SER2014; Gulsvik; SBPT; Ip; Quanjer; Wang; Dockery and more
Technical Specification
| Spirotrac software | |
|---|---|
| Product | Vitalograph Spirotrac |
| Model | 7000 |
| QA/GMP Standards | ISO 13485, FDA 21 CFR 820, SOR/98-282 & JPAL, MDSAP |
| Device Classification | II (US)/IIa (EU) |