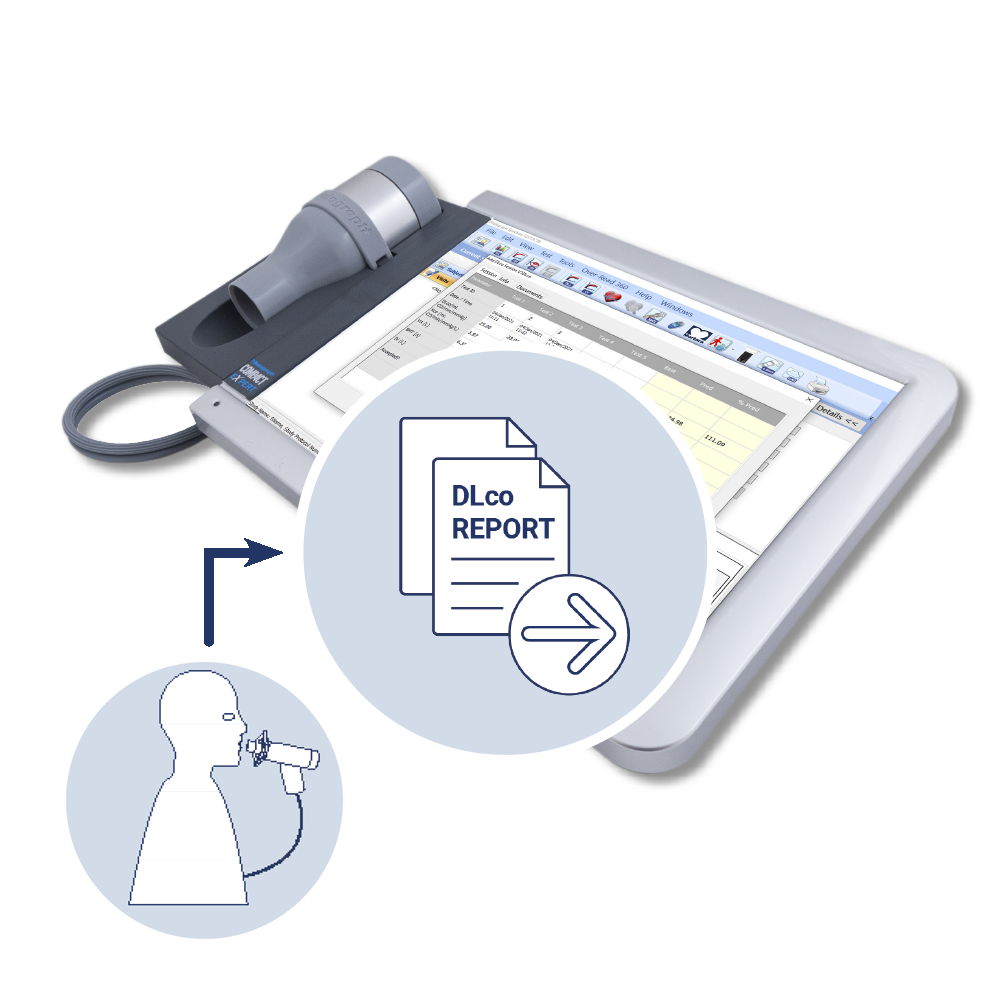
DLco Data Capture
Quality Assured DLco Data in your Centralized Respiratory Dataset
Assessing the diffusing capacity of the lungs for carbon monoxide (DLco) is one of the most clinically valuable tests of lung function and a commonly used endpoint in respiratory clinical trials. The Vitalograph® DLco solution enables reports from sites’ own equipment to be uploaded to the COMPACT™ Medical Workstation for inclusion alongside other study data, in a centralized respiratory data set. This approach minimises study device rental costs and equipment storage for sites.
DLco reports are manually uploaded to the COMPACT Medical Workstation by qualified site staff and sent to a central Vitalograph database. For quality assurance, Vitalograph clinical specialists over-read DLco data, providing accessible results and feedback to continuously improve site data, compliance and performance throughout the study.
- Centralized respiratory dataset to simplify data management.
- No additional cost incurred for rental of DLco equipment where a site’s own DLco equipment is used.
- All training, support and equipment required for upload are provided by Vitalograph.
- Expert over-reading service for quality assured data.
- Accessible data for site and sponsor monitoring.
- Key opinion leaders (KOLs) on hand to advise and support with study design.
Technical Specification
| Vitalograph COMPACT | |
|---|---|
| Model Number | 6600 |
| Dimensions | 375mm x 235mm x 110mm |
| Weight | Net: 2.5kg |
| Communications | USB x minimum 6, Serial x 2, Ethernet x 2, DVI, WiFi |
| QA/GMP Standards | ISO 13485, FDA 21 CFR 820, SOR/98-282, JPAL, MDSAP |
| Voltage/Frequency | 110-240 V; approximately 50/60 Hz |
| Performance Standards/Guidelines met or exceeded by the Vitalograph COMPACT Expert | ISO 23747, ISO 26782, ATS/ERS 2019 |
| Spirometry Specifications | |
| Flow detection principle | Fleisch type pneumotacograph |
| Back pressure | Less than 0.1kPa/L/second @ 14L/s, complies with ISO26782:2009 |
| Volume accuracy | ±2.5% or 0.05L |
| Flow accuracy | Flow ±10% or 0.3 L/s |